Dr. Gopi Patel recalls how powerless she felt when New York’s Mount Sinai Hospital overflowed with COVID-19 patients in March.
Guidance on how to treat the disease was scant, and medical studies were being performed so hastily they couldn’t always be trusted.
“You felt very helpless,” said Patel, an infectious disease doctor at the hospital. “I’m standing in front of a patient, watching them struggle to breathe. What can I give them?”
While there is still no simple answer to that question, a lot has changed in the six months since an entirely new coronavirus began sweeping the globe.
Doctors say they’ve learned enough about the highly contagious virus to solve some key problems for many patients. The changes could be translating into more saved lives, although there is little conclusive data.
Nearly 30 doctors around the world, from New Orleans to London to Dubai, told Reuters they feel more prepared should cases surge again in the fall.
“We are well-positioned for a second wave,” Patel said. “We know so much more.”
Doctors like Patel now have:
*A clearer grasp of the disease’s side effects, like blood clotting and kidney failure
*A better understanding of how to help patients struggling to breathe
*More information on which drugs work for which kinds of patients.
They also have acquired new tools to aid in the battle, including:
*Widespread testing
*Promising new treatments like convalescent plasma, antiviral drugs and steroids
*An evolving spate of medical research and anecdotal evidence, which doctors share across institutions, and sometimes across oceans.
Despite a steady rise in COVID-19 cases, driven to some extent by wider testing, the daily death toll from the disease is falling in some countries, including the United States. Doctors say they are more confident in caring for patients than they were in the chaotic first weeks of the pandemic, when they operated on nothing but blind instinct.
In June, an average of 4,599 people a day died from COVID-19 worldwide, down from 6,375 a day in April, according to Reuters data.
New York’s Northwell Health reported a fatality rate of 21% for COVID-19 patients admitted to its hospitals in March. That rate is now closer to 10%, due to a combination of earlier treatment and improved patient management, Dr. Thomas McGinn, director of Northwell’s Feinstein Institutes for Medical Research, told Reuters.
“I think everybody is seeing that,” he said. “I think people are coming in sooner, there is better use of blood thinners, and a lot of small things are adding up.”
Even nuts-and-bolts issues, like how to re-organize hospital space to handle a surge of COVID-19 patients and secure personal protective equipment (PPE) for medical workers, are not the time-consuming, mad scrambles they were before.
“The hysteria of who’d take care of (hospital staff) is not there anymore,” said Dr. Andra Blomkalns, head of emergency medicine at Stanford Health Care, a California hospital affiliated with Stanford University. “We have an entire team whose only job is getting PPE.”
To be sure, the world is far from safe from a virus that continues to rage. It is expected to reach two grim milestones in the next several days: 10 million confirmed global infections and 500,000 deaths. As of Thursday evening, more than 9.5 million people had tested positive for the coronavirus, and more than 483,000 had died, according to Reuters data. The United States remains the epicenter of the pandemic, and cases are rising at an alarming pace in states like Arizona, Florida and Texas.
There is still no surefire treatment for COVID-19, the disease caused by the new virus, which often starts as a respiratory illness but can spread to attack organs including the heart, liver, kidneys or central nervous system. Scientists are at least months away from a working vaccine.
And while medical knowledge has improved, doctors continue to emphasize that the best way for people to survive is to avoid infection in the first place through good hygiene, face coverings and limited group interaction.
Dr. Ramanathan Venkiteswaran, medical director of Aster Hospitals in the United Arab Emirates, said COVID-19 will likely result in permanent changes in medicine and for the general public on “basic things like social distancing, wearing of masks and hand washing.”
LEARNING ON THE FLY
In the medical field, change can be slow, with years-long studies often needed before recommendations are altered. But protocols for COVID-19 have evolved at lightning speed.
In Brazil, São Paulo-based Hospital Israelita Albert Einstein, one of the country’s leading private hospital networks, has updated its internal guidelines for treating coronavirus patients some 50 times since the outbreak began earlier this year, according to Dr. Moacyr Silva Junior, an infectious disease specialist at the center. Those guidelines govern questions such as which patients are eligible for which drugs, how to handle patients with breathing problems, and the use of PPE like masks, gowns and gloves.
“In only three months, a resounding amount of scientific work on COVID-19 has been published,” he said.
At Stanford Health Care, treatment guidelines changed almost daily in the early weeks of the pandemic, Blomkalns said. She described a patchwork approach that began by following guidelines established by the U.S. Centers for Disease Control and Prevention, then modifying them to reflect a shortage of resources, and finally adding new measures not addressed by the CDC, such as how to handle pregnant healthcare workers.
The new coronavirus has been particularly vexing for doctors because of the many and often unpredictable ways it can manifest. Most people infected experience only mild flu-like symptoms, but some can develop severe pneumonia, stroke and neurological disease. Doctors say the biggest advance so far has been understanding how the disease can put patients at much higher risk for blood clots. Most recently, doctors have discovered that blood type might influence how the body reacts to the virus.
“We developed specific protocols, such as when to start blood thinners, that are different from what would be done for typical ICU patients,” said Dr. Jeremy Falk, pulmonary critical care specialist at Cedars-Sinai Medical Center in Los Angeles.
Around 15% of COVID-19 patients are at risk of becoming sick enough to require hospitalization. Scientists have estimated that the fatality rate could be as high as 5%, but most put the number well below 1%. People with the highest risk of severe disease include older adults and those with underlying health conditions like heart disease, diabetes and obesity.
While rates of COVID-19 infection have recently been rising in many parts of the United States, the total number of U.S. patients hospitalized with COVID-19 has been steadily falling since a peak in late April, according to the CDC.
Many hospitals report success with guidelines for “proning” patients – positioning them on their stomachs to relieve pressure on the lungs, and hopefully stave off the need for mechanical ventilation, which many doctors said has done more harm than good.
“At first, we had no idea how to treat severely ill patients when we (ventilate),” said Dr. Satoru Hashimoto, who directs the intensive care division at Kyoto Prefectural University of Medicine in Japan. “We treated them in the fashion we treated influenza,” only to see those patients suffer serious kidney, digestive and other problems, he said.
Hospitals say increased coronavirus testing – and faster turnaround times to get results – are also making a difference.
“What has really helped us triage patients is the availability of rapid testing that came on about six weeks ago,” said Falk of Cedars-Sinai. “Initially, we had to wait two, three or even four days to get a test back. That really clogged up the COVID areas of the hospital.”
Faster, wider testing also helps conserve PPE by identifying the negative patients around whom doctors don’t have to wear as much gear, said Dr. Saj Patel, who treats non-critical patients at the University of California San Francisco Medical Center. “You can imagine how much PPE we burned through” waiting for test results, he said.
Hospitals around the world acted early to restructure operations, including floor layouts, to isolate coronavirus patients and reduce exposure to others. It wasn’t always smooth, but doctors say they’re figuring out how to do it more efficiently.
“Our hospital infrastructure, and the way that we … manage people coming through the door is a lot slicker than it was earlier in the epidemic,” said Dr. Tom Wingfield, a clinical lecturer at the Liverpool School of Tropical Medicine in Liverpool, England.
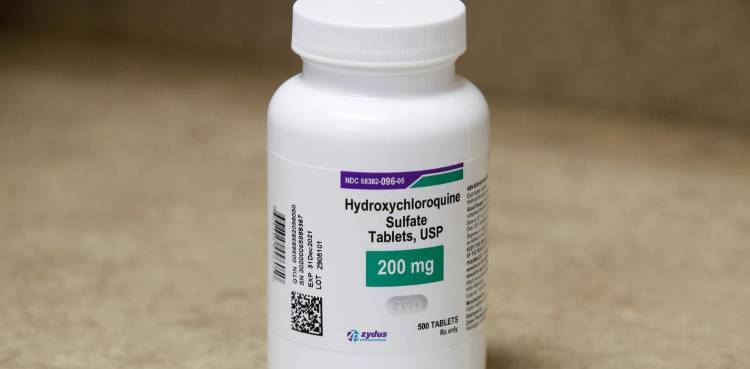
‘THE PRESIDENT’S DRUG’
Hospitals said some of their early hunches about best treatments for COVID-19 patients ended up being wrong. Case in point: use of the anti-malaria pill hydroxychloroquine.
It gained attention in March, when U.S. President Donald Trump began publicly touting it. Early reports showed the drug could have some benefit, and hospitals, desperate for solutions, started giving it to critically sick patients. But subsequent trial data have told a different story, suggesting the drug is not effective for treatment or prevention, and might even cause harm. Other clinical trials of the drug are still underway.
Dr. Mangala Narasimhan, regional director of critical care at Northwell Hospital in New York, recalled the uncertainty around hydroxychloroquine. The hospital used it early on, but stopped after the negative studies were published. “That was one of our mainstays of treatment in the beginning,” Narasimhan said. “We didn’t have anything else.”
Trump’s loud support for the drug turned the medical debate into a political one. That happened in Brazil, too, when far-right President Jair Bolsonaro fiercely supported hydroxychloroquine. Hospital Sírio-Libanês, in São Paulo, is one of the many hospitals around the globe that have now abandoned it.
Some patients at Sírio-Libanês refused to be part of clinical trials involving what they called the “president’s drug,” said Dr. Mirian Dal Ben, an epidemiologist there, while others demanded to be treated with it.
The lingering questions about use of hydroxychloroquine highlight the hazards of quickly moving science. Hospitals normally rely on fully vetted research published by prominent medical journals like the Lancet and the New England Journal of Medicine to flag important medical findings. But as the pandemic built, so did the number of so-called “pre-print” studies that have not been peer-reviewed.
The Montpellier University Hospital in southern France used hydroxychloroquine on severely ill patients until the government banned the substance in May.
“I have no major regrets when looking back on the decisions that we took,” said Dr. Jacques Reynes, head of infectious and tropical diseases. “But I would say that, at the beginning, we were somewhat in a fog.”
USING WHAT’S AT HAND
But even if hydroxychloroquine looks unlikely as an effective COVID-19 treatment, hospitals continue to try new medications – both by repurposing older drugs and exploring novel therapies. Patients are being enrolled in hundreds of coronavirus clinical trials launched in the past three months.
Many hospitals said they are seeing success with the use of plasma donated by survivors of COVID-19 to treat newly infected patients.
People who survive an infectious disease like COVID-19 are generally left with blood containing antibodies, which are proteins made by the body’s immune system to fight off a virus. The blood component that carries the antibodies, known as convalescent plasma, can be collected and given to new patients.
Early results from a study at New York’s Mount Sinai Hospital found that patients with severe COVID-19 who were given convalescent plasma were more likely to stabilize or need less oxygen support than other similar hospital patients. But results from other studies have been mixed, and doctors still await findings from a rigorously-designed trial. And availability of plasma varies between regions.
At Henry Ford Hospital in Detroit, Michigan, “anecdotally everyone can provide stories” of the benefits of plasma, said Dr. John Deledda, the hospital’s chief medical officer.
But in rural New Mexico, hospitals that care for largely underserved populations struggle to find it. “There’s a limited number of blood centers” that can provide plasma, said Valory Wangler, chief medical officer at Rehoboth McKinley Christian Health Care Services, in Gallup, New Mexico. Until trial data is more conclusive, plasma is “not something we’re pursuing actively,” she said.
Dr Abdullatif al-Khal, head of infectious diseases at Qatar’s Hamad Medical Corporation and a co-chair of the country’s pandemic preparedness team, said he saw patients improve after he started using donated plasma early in the course of COVID-19 before the patients deteriorated.
Qatar is also assessing a steroid known as dexamethasone to treat COVID-19. But Khal says he wants to wait for publication of clinical data behind a recent UK study suggesting that the steroid reduced death rates by around a third among the most severely ill COVID-19 patients.
In patients with severe COVID-19, the immune system can overreact, triggering a potentially harmful cascade. Steroids are an older class of drugs that suppress that inflammatory response. But they can also make it easier for other viral or bacterial infections to take hold – making doctors leery of their use in a hospital setting or in patients with early-stage COVID-19.
Some countries, including Bahrain and the United Arab Emirates, reported using HIV drugs lopinavir and ritonavir with some success. Clinical trials, though, have suggested little benefit, and they aren’t widely used in the United States.
MIDNIGHT DELIVERY
Many of the doctors who spoke with Reuters were bullish on the use of remdesivir, the only drug so far shown to be effective against the coronavirus in a rigorous clinical trial. The antiviral developed by California-based Gilead Sciences Inc (GILD.O) was shown to reduce the length of hospital stays for COVID-19 patients by about a third, but hasn’t been proven to boost survival.
Remdesivir is designed to disable the mechanism by which certain viruses, including the new coronavirus, make copies of themselves and potentially overwhelm their host’s immune system.
It is available under emergency approvals in several countries, including the United States. But Gilead’s donated supplies are limited, and distribution and availability are uneven.
Dr. Andrew Staricco, chief medical officer at McLaren Health Care, which operates 11 hospitals across Michigan, recalls the urgency to obtain remdesivir early on. He got an email from Michigan’s health department on May 9, a week after the U.S. Food & Drug Administration authorized the drug for use in treating COVID-19. The health department said it had received a small batch from the federal government, and planned to dole it out to local hospitals based on need. Staricco wrote back, saying he had 15 to 18 critically ill patients, but was given enough to treat just four.
The drug was so precious, he said, that state police troopers were responsible for transporting it to the hospital – which they did, dropping it off around 1 a.m. the next morning.
Health officials originally directed remdesivir for use on the most critically ill patients. But doctors later found they got the best results administering it earlier.
“We started finding that, actually, the sooner you get treated with it, the better,” Staricco said. “We’ve revisited our criteria for giving it to patients three different times.”
Data on the drug, he said, is still scarce. But his anecdotal observations on the benefits of early treatment were echoed by several U.S. doctors.
‘COPY-CATTING’
Gilead on Monday said it aims to manufacture another 2 million courses of remdesivir this year, but did not comment on how it plans to distribute, or sell, those supplies for use by hospitals. The company has licensed the antiviral to several generic drugmakers, who will be allowed to sell the medication in over 100 low-income nations.
Although much about the coronavirus remains unknown, a key reason hospitals say they now are more prepared owes to teamwork.
Many doctors described a kind of unofficial network of information sharing.
In hard-hit Italy, Dr. Lorenzo Dagna of the IRCCS San Raffaele Scientific Institute in Milan, organized conference calls with institutions in the United States and elsewhere to share experiences and anecdotes treating COVID-19 patients.
McLaren’s Staricco said the Michigan hospital chain adopted its policy on use of blood thinners by looking at peers at Detroit Medical Center and Vanderbilt University Medical Center.
As more institutions put their guidelines online, he said, there was “lots of copy-catting going on.”
The post Doctors see hope in new COVID-19 treatments appeared first on ARY NEWS.
Comments